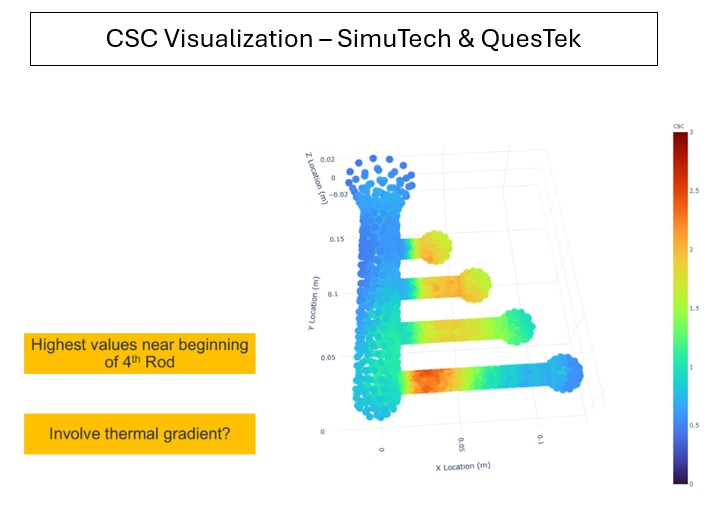
Introduction to Medical Device Development Simulation
Medical device manufacturers face growing pressure to innovate quickly while meeting increasing safety and performance expectations. Traditional prototype-only methods — build, test, refine — are no longer enough to keep pace with evolving technologies and their impact on clinical practices. Simulation technology is now a critical tool for evaluating new design concepts, improving device capabilities, and accelerating development timelines.
Why Medical Device Companies Are Turning to Simulation
Simulation offers several advantages that physical testing alone cannot deliver. It allows engineers to explore scenarios that would be unsafe or impossible to evaluate clinically. Instead of relying solely on prototypes, teams can review large numbers of design variations quickly and understand how each option performs across the entire solution domain—not just at several, specific test conditions.
In terms of exploring what can’t be tested physically, simulation makes it possible to predict pressure, flow, stress, heat transfer, and electrical behavior in greater detail than can be measured physically, providing earlier insight into performance risks and design tradeoffs before costly validation and clinical testing begins.

Evaluating Multiple Medical Device Designs Rapidly
With incorporating simulation into development, teams can numerically evaluate dozens or hundreds of variations without building new hardware, accelerating development cycles significantly.
Gaining Full Visibility Into Device Behavior
Simulation provides comprehensive data across the entire geometry and physics environment, revealing insights that physical testing may not be able to reveal.
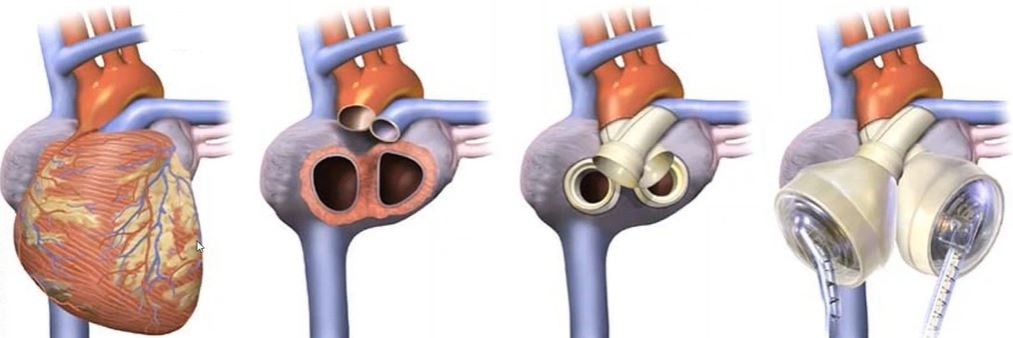
Real-World Example: Preventing Blood Clots in an Artificial Heart Pump
During early animal testing of an artificial heart blood pump, clinicians observed thrombus forming on the right impeller. The left impeller was clean without any evidence of thrombus formation. Using computational fluid dynamics, SimuTech modeled both impellers and found regions of low shear stress on several surfaces of the right impeller. These areas closely matched the thrombus locations found in testing. Using this insight, the design was refined to reduce low-shear regions and decrease the likelihood of thrombus formation.
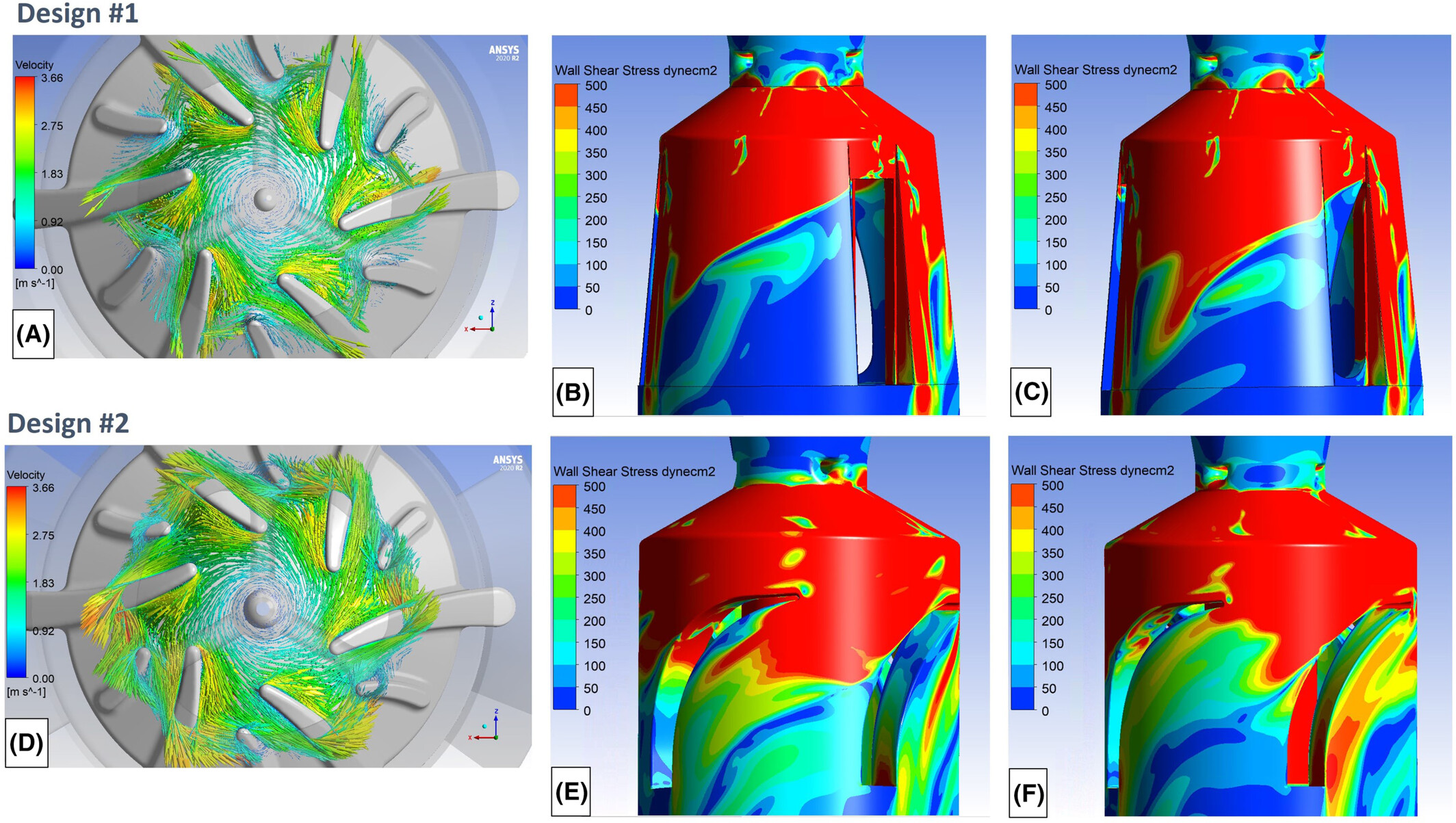
Choosing the Right Medical Device Development Simulation Approach
SimuTech evaluates the specific engineering challenges of each medical device development simulation project to determine whether CFD, FEA, electromagnetics, optics, or a multiphysics combination is needed. This ensures that the selected tools align with the device’s requirements and provide accurate, actionable insights.
The Future of Simulation in Healthcare
Two major developments are shaping the future of medical device simulation:
Patient-Specific Modeling
Using MRI or CT data, engineers can simulate device performance within a specific patient’s anatomy. This supports personalized medicine, including pediatric applications and specialized interventions where anatomy or physical properties vary significantly across different patient populations.
Multiphysics Human Modeling
Advanced simulations now combine structural mechanics, electrical activation, and fluid dynamics to model organ behavior—such as the motion and electrical stimulation of the human heart. These tools can be used to create a deeper understanding of how new medical devices interact with the human body.
Business Impact and Market Success
Simulation offers measurable ROI. For example, two oxygenators developed using simulation-guided design went on to become leading market products, with one achieving the top global market position. Modeling enabled rapid iteration, optimized flow paths, and improved performance, contributing to strong commercial success.
Advice for Teams New to Medical Device Development Simulation
Start small with a targeted technical challenge that can produce clear results. Use early wins to demonstrate the value of computational modeling, build internal confidence, and expand the use of simulation across the development lifecycle. Medical device development simulation is most effective when used throughout the product development cycle from providing early design guidance through supporting product introduction and continuing design improvements.
Final Thoughts
Simulation has become a core part of modern medical device engineering. It enhances product safety and performance, speeds development, and reveals insights that physical testing alone cannot. SimuTech Group supports teams across the full innovation spectrum, helping bring safer and more effective devices to patients faster.