Introduction
Developing a medical device is as much about execution as it is about innovation; even the most promising concept must first prove its ability to perform in real-world scenarios without putting patients at risk. Safety, reliability, and regulatory compliance are non-negotiables. The path from concept to clinical use demands rigorous design validation to ensure that devices function as intended in the body, withstand real-world conditions, and meet stringent regulatory standards. That’s where engineering simulation makes a measurable impact.
Simulation technology accelerates medical device development by enabling engineers to predict performance, test safety limits, and fine-tune product design, all before a physical prototype is ever produced. From cardiovascular implants to orthopedic tools, SimuTech Group empowers healthcare innovators with advanced multiphysics modeling and simulation consulting services.
Streamlining Medical Device Design with Simulation
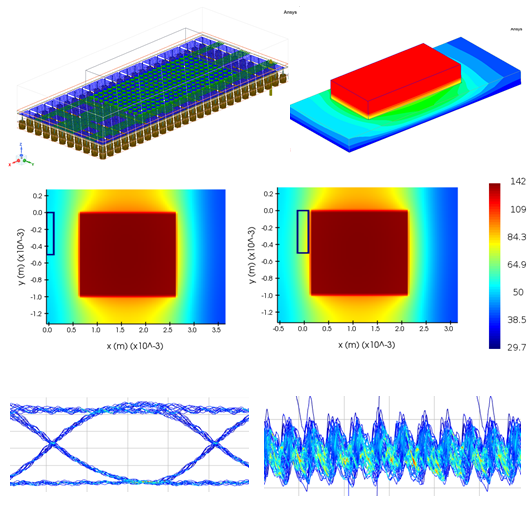
Medical device simulation allows engineers to virtually test a product’s behavior under physiological conditions, significantly reducing reliance on expensive and time-consuming fabrication and testing of physical prototypes. Using Ansys software, with the support of SimuTech Group simulation consultants, clients can simulate:
- Blood flowing through rotary blood pumps to ensure adequate perfusion while minimizing the potential for blood damage.
- Thermal stresses in surgical cooling devices for precision control and patient safety.
- Mechanical integrity of orthopedic implants and spinal devices under varied loads and patient movements.
Simulation makes it easier to evaluate design iterations quickly, understand material behaviors, and avoid late-stage failures that can derail product development.
Accelerating Regulatory Approval with Predictive Insight
Both the FDA and global regulatory bodies are increasingly embracing simulation as part of the regulatory submission process. Tools like Finite Element Analysis (FEA), Computational Fluid Dynamics (CFD), Electromagnetics (EMAG) modeling can be used to help demonstrate a medical device’s safety, efficacy, and durability.
For example, SimuTech engineers routinely support:
- Preliminary blood flow-related biocompatibility and thermal modeling.
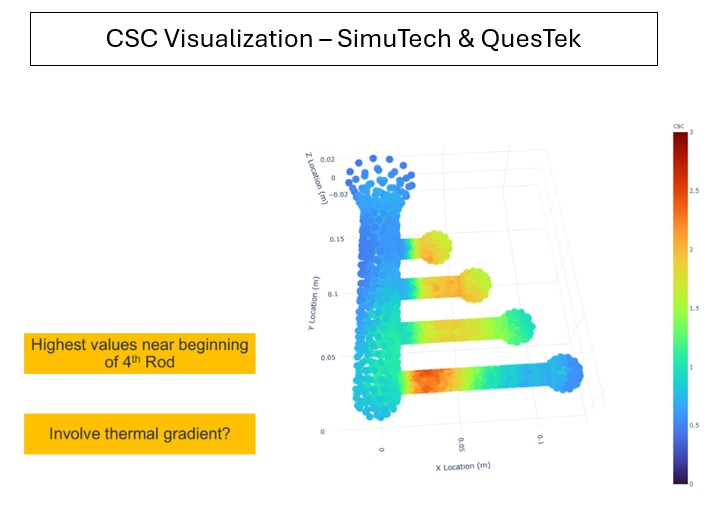
- Predicting wear and fatigue simulations for long-term implant performance.
- Assessing the likelihood of blood hemolysis and platelet activation in cardiovascular devices.
Simulation is a strategic asset in securing faster regulatory clearance and reducing clinical risk.
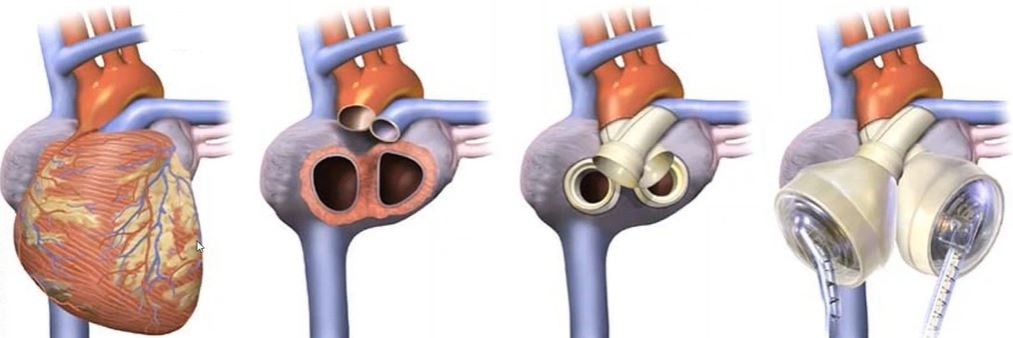
Patient-Specific Modeling: Personalizing Surgical Outcomes
Patient-specific computational modeling is transforming medical device simulation, product development, and surgical planning. SimuTech Group uses patient imaging data to reconstruct 3D models of a patient’s anatomy, enabling:
- Customized device design and sizing
- Pre-surgical procedural planning
- Evaluation of new interventions under simulated clinical scenarios
This personalized approach is already being used in applications ranging from contact lenses to intraocular surgeries, spinal implants, and catheter-based treatments.
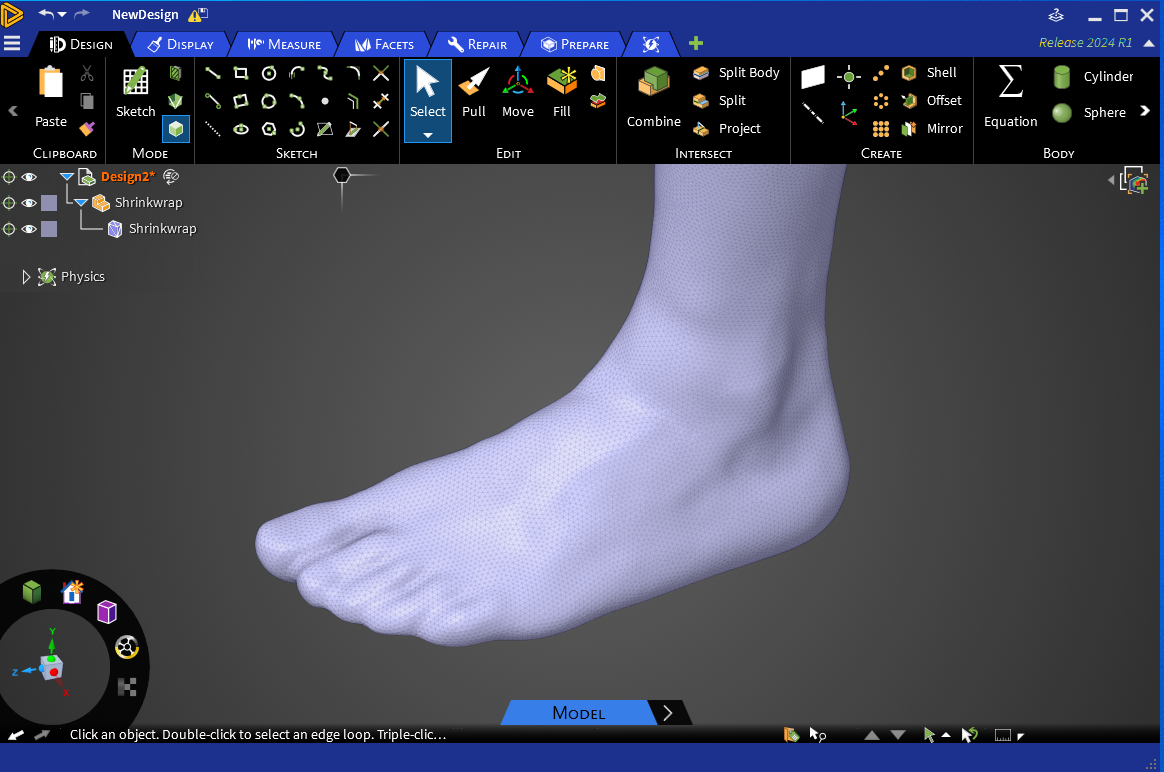
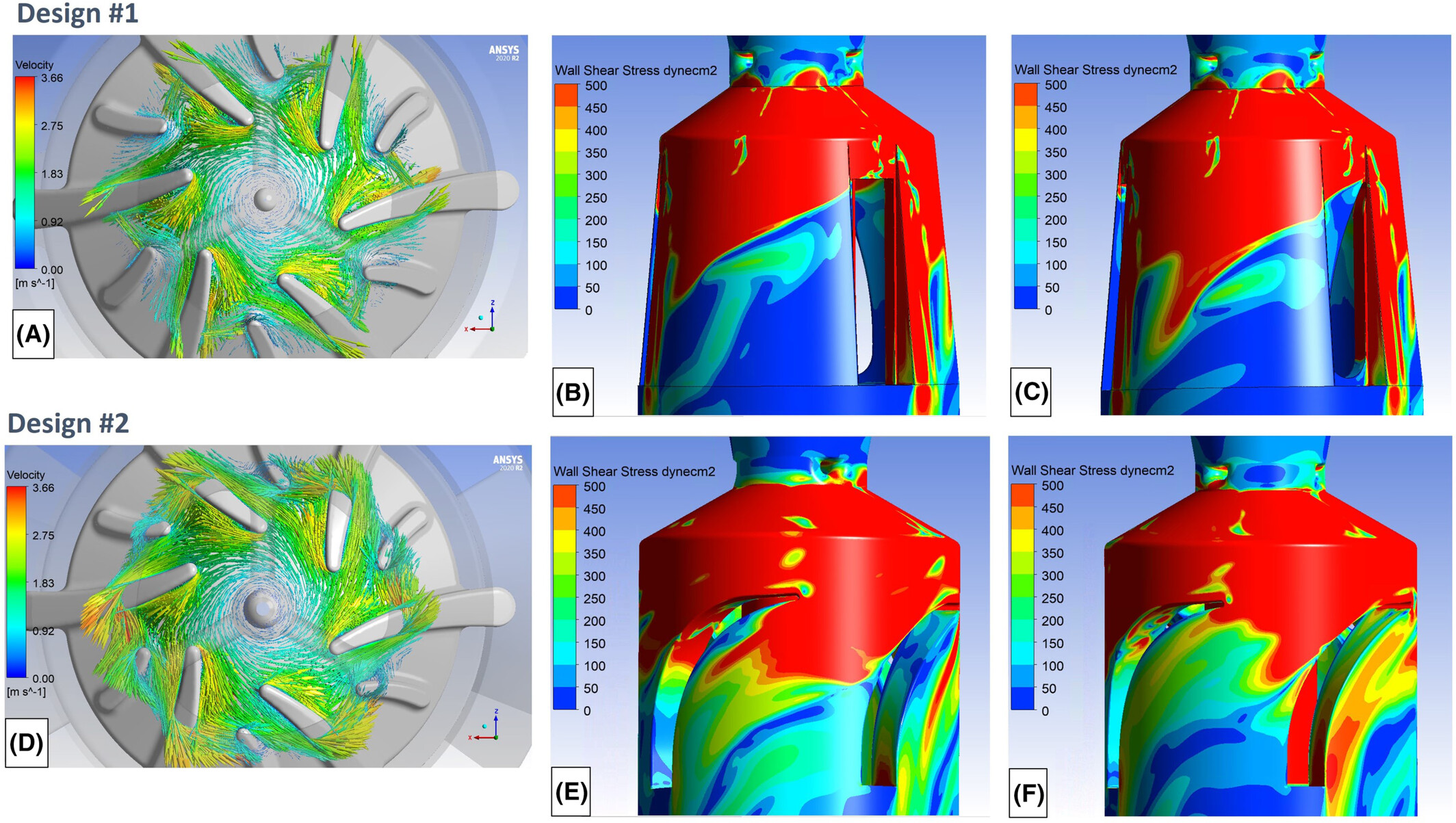
Multiphysics Simulation for Complex Medical Systems
Modern medical devices often combine mechanical, electrical, thermal, and fluidic components. SimuTech’s engineering staff leverages Ansys multiphysics capabilities to predict the interaction between these physics to design:
- MEMS drug delivery systems with precise control over thermal diffusion and dosing.
- Ultrasound imaging systems with optimized signal pathways.
- Miniaturized renal assist devices with uniformly distributed flow to achieve the desired mass transport while also ensuring sufficient structural integrity during clinical use.
These insights help design smaller, more efficient, and safer devices that can improve patient care.
Supporting Innovation Across Healthcare Disciplines
SimuTech Group brings decades of experience supporting medical device simulation in disciplines such as:
- Cardiovascular: Ventricular assist devices, blood oxygenators, and catheters
- Orthopedics: Spinal implants, cooling devices, and imaging system structures
- Respiratory: Lung particle deposition, airway devices, and surgical airflow control
- Ophthalmology: Replacement lenses, corneal simulations, and VR-enabled surgical simulators
Each solution is customized to the client’s goals, regulatory needs, and time-to-market requirements.
Meeting Compliance, Maximizing Confidence
Meeting regulatory expectations is a team effort. SimuTech Group helps organizations prepare documentation for FDA submissions, align with ISO standards, and respond to validation requests with clarity and confidence.
Simulation delivers actionable evidence for regulators and stakeholders alike, whether demonstrating electromagnetic safety in surgical tools, quantifying temperature rise in tissue-adjacent electronics, assessing biocompatibility, modeling fatigue, ensuring well-distributed flow, and more.
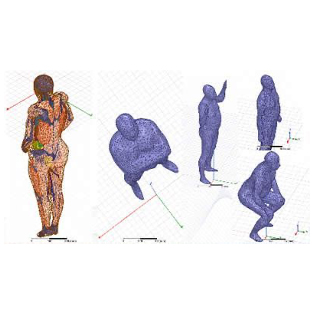
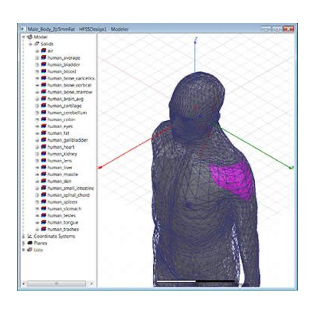
From Concept to Commercialization
With the ability to run simulations early in the development cycle, medical device companies gain significant advantages:
- Reduced physical testing and prototyping costs
- Faster design iterations and shorter time to market
- More robust, clinically effective products
- Greater confidence in meeting regulatory requirements
At SimuTech Group, we enable this transformation through expert consulting, simulation mentoring, and tailored support from concept to regulatory submission.
Simulation is the Future of Medical Innovation
As demand grows for more complex and precise medical devices, the need for advanced engineering tools grows exponentially. Simulation is a core enabler of innovation, patient safety, and regulatory success.
SimuTech Group remains at the forefront of this shift, providing the healthcare sector with trusted Medical Device Consulting and simulation expertise that bridges the gap between brilliant ideas and breakthrough products.
Maximize Your Confidence in Every Medical Device Submission
Contact us today to talk with one of our biomedical simulation experts and start the conversation.